
Thus, T g demarks embrittlement and can dictate morphological evolution in the active layer of electronic devices, thereby impacting mechanical and electrical performance. Annealing above T g can lead to cold crystallization 8, 9, enhancement of liquid crystalline order 10, or stronger phase separation with another additive or polymer 11, 12, 13.
The kinetics of morphological evolution with processing or under operation are limited by chain diffusion or segmental motion, which depends on the temperature with respect to T g.

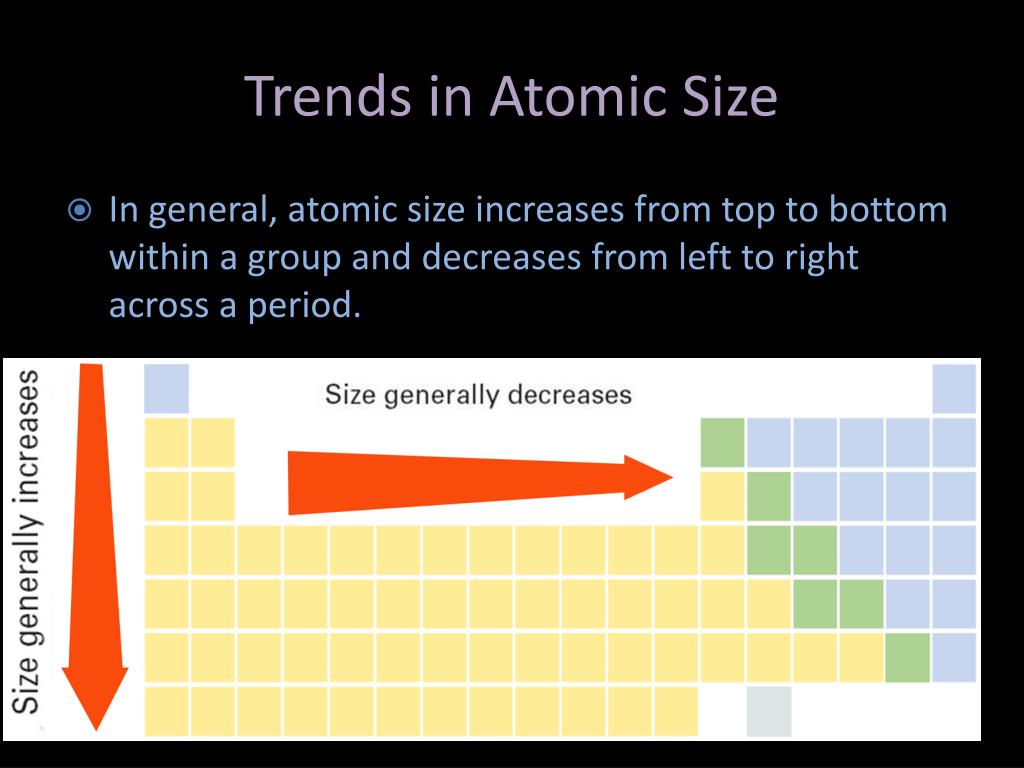
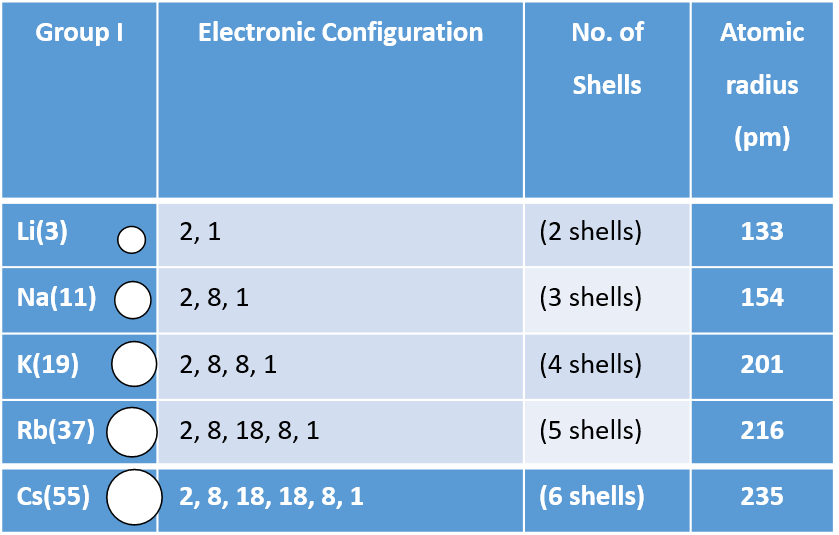
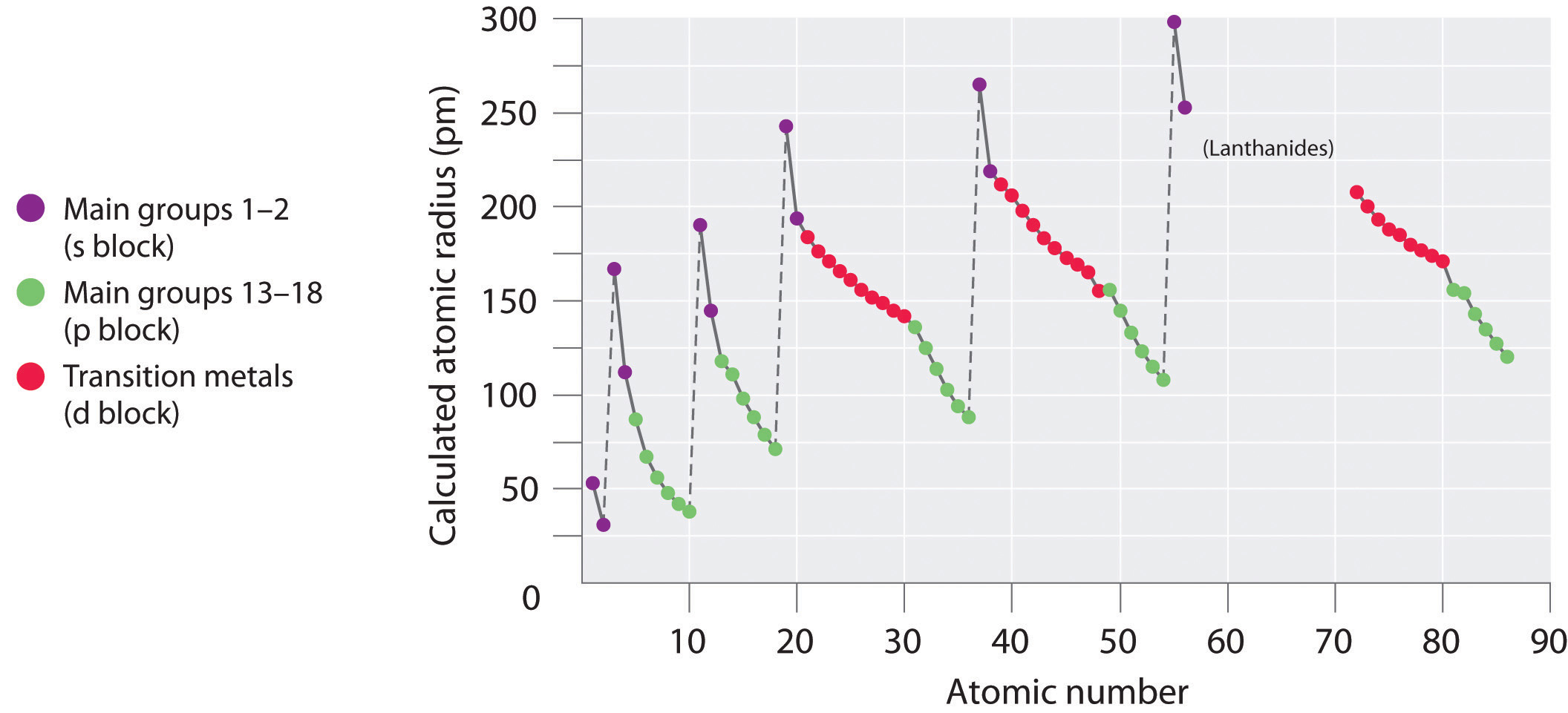
Furthermore, solution-processing of conjugated polymers for integration into various electronic devices 5, 6, 7 results in morphologies that are far from equilibrium and which tend to evolve with time. Therefore, one of the key design requirements for soft or stretchable electronics is to ensure that T g lies below room temperature 3, 4. At higher temperatures, the storage modulus decreases orders of magnitude to ~10 MPa for a semicrystalline polymer, or ~1 MPa for an entangled amorphous polymer 1, 2. Below T g, the storage moduli of conjugated polymers are on the order of one GPa, making the materials too stiff and too brittle for flexible devices 1. The result is a steady increase in the effective nuclear charge and a steady decrease in atomic size ( Figure 4.3.5).įigure 4.3.5: The Atomic Radius of the Elements.The glass transition temperature ( T g) of conjugated polymers governs chain dynamics and mechanical properties. Similarly, as we proceed across the row, the increasing nuclear charge is not effectively neutralized by the electrons being added to the s and p orbitals. Consequently, beryllium is significantly smaller than lithium. This means that the effective nuclear charge experienced by the s electrons in beryllium is between +1 and +2 (the calculated value is +1.66). Electron density diminishes gradually with increasing distance, which makes it impossible to draw a sharp line marking the boundary of an atom.įigure 1: Plots of Radial Probability as a Function of Distance from the Nucleus for \ce.) In contrast, the two s electrons in beryllium do not shield each other very well, although the filled s 2 shell effectively neutralizes two of the four positive charges in the nucleus. This point is illustrated in Figure 4.3.1 which shows a plot of total electron density for all occupied orbitals for three noble gases as a function of their distance from the nucleus. Recall that the probability of finding an electron in the various available orbitals falls off slowly as the distance from the nucleus increases. In this chapter, we will discuss how atomic and ion “sizes” are defined and obtained.
As a result, atoms and ions cannot be said to have exact sizes however, some atoms are larger or smaller than others, and this influences their chemistry. To understand periodic trends in atomic radii.Īlthough some people fall into the trap of visualizing atoms and ions as small, hard spheres similar to miniature table-tennis balls or marbles, the quantum mechanical model tells us that their shapes and boundaries are much less definite than those images suggest.


 0 kommentar(er)
0 kommentar(er)
